Precision Medicine in Endometriosis: Emerging Disease-Modifying Pathways
Endometriosis affects at least 1 in 10 women worldwide and is a leading cause of chronic pelvic pain, infertility, and reduced quality of life. Yet despite its prevalence and burden, the disease remains severely underfunded, receiving less than 1% of NIH funding in 2024.
Most available treatments today focus on symptom management, not the underlying disease biology. Hormonal therapies and contraceptives can reduce pain for some patients, but they do not halt disease progression. Surgery remains the gold standard for lesion removal, yet it is invasive and recurrence is common.
To meaningfully change outcomes, the field needs disease-modifying therapies, approaches that intervene at the molecular drivers of endometriosis itself. In this mini-series, we explored five emerging biological pathways that could help move endometriosis care beyond symptom control and toward precision medicine.
Why Disease Modification Matters in Endometriosis
Endometriosis is not a single disease with a single mechanism. It is a biologically complex, heterogeneous condition involving inflammation, immune dysfunction, fibrosis, angiogenesis, hormonal signaling, and pain sensitization. This complexity helps explain why one-size-fits-all treatments have fallen short.
Targeting the specific molecular pathways driving lesion growth and persistence opens the door to more effective, durable, and personalized care—without relying solely on systemic hormone suppression.
Below, we highlight five pathways currently under investigation for their disease-modifying potential.
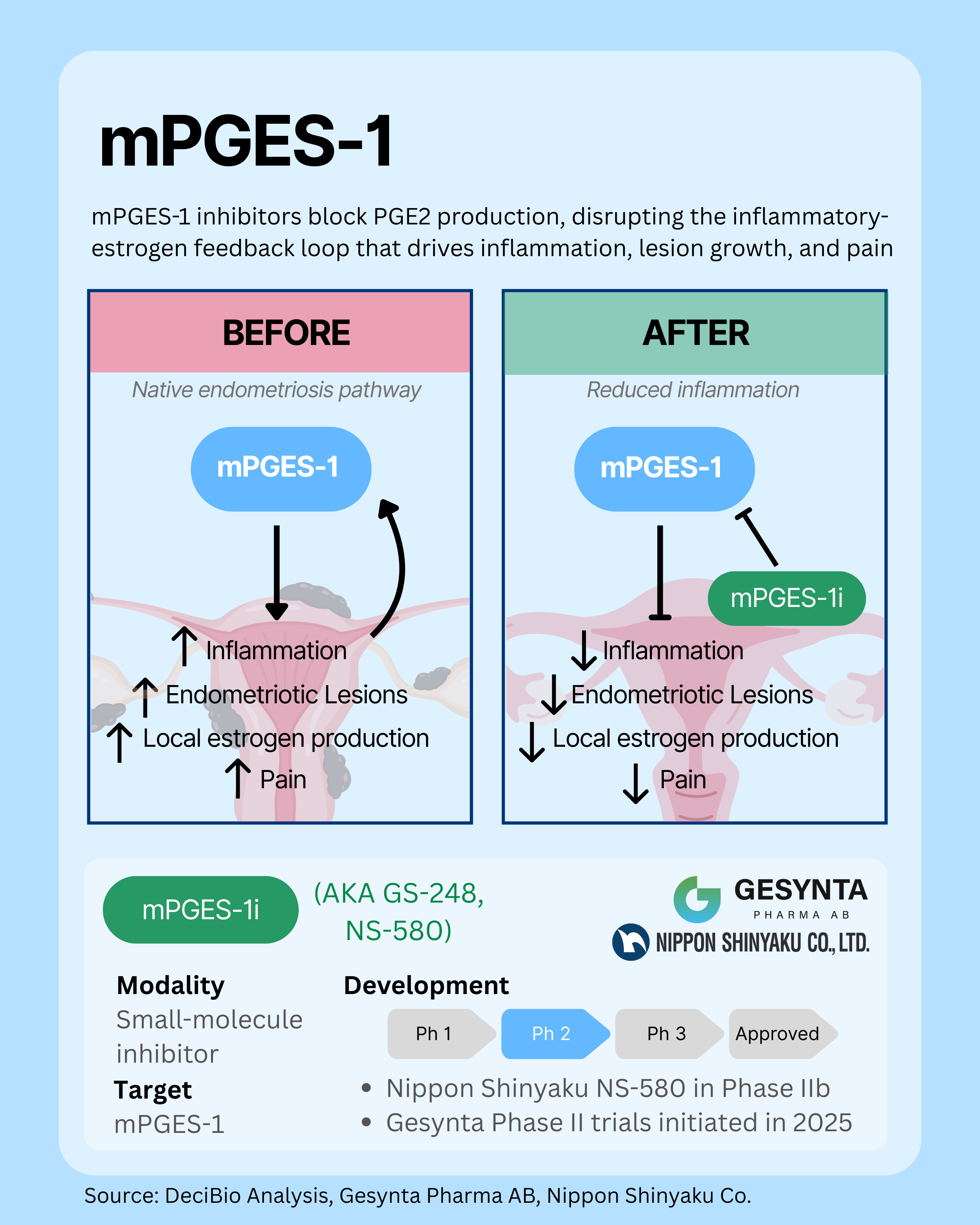
1. mPGES-1: Targeting Prostaglandin-Driven Inflammation at the Source
mPGES-1 is a key enzyme in the production of prostaglandin E2, a signaling molecule that drives pain, inflammation, and endometriotic lesion growth through a feedback loop with local estrogen production. By selectively inhibiting mPGES-1, new therapies could block PGE2 synthesis at its source, dampening inflammation, actively reducing lesion formation, and addressing underlying disease rather than just treating symptoms. Unlike traditional hormone therapies, this approach does not require systemic estrogen suppression.
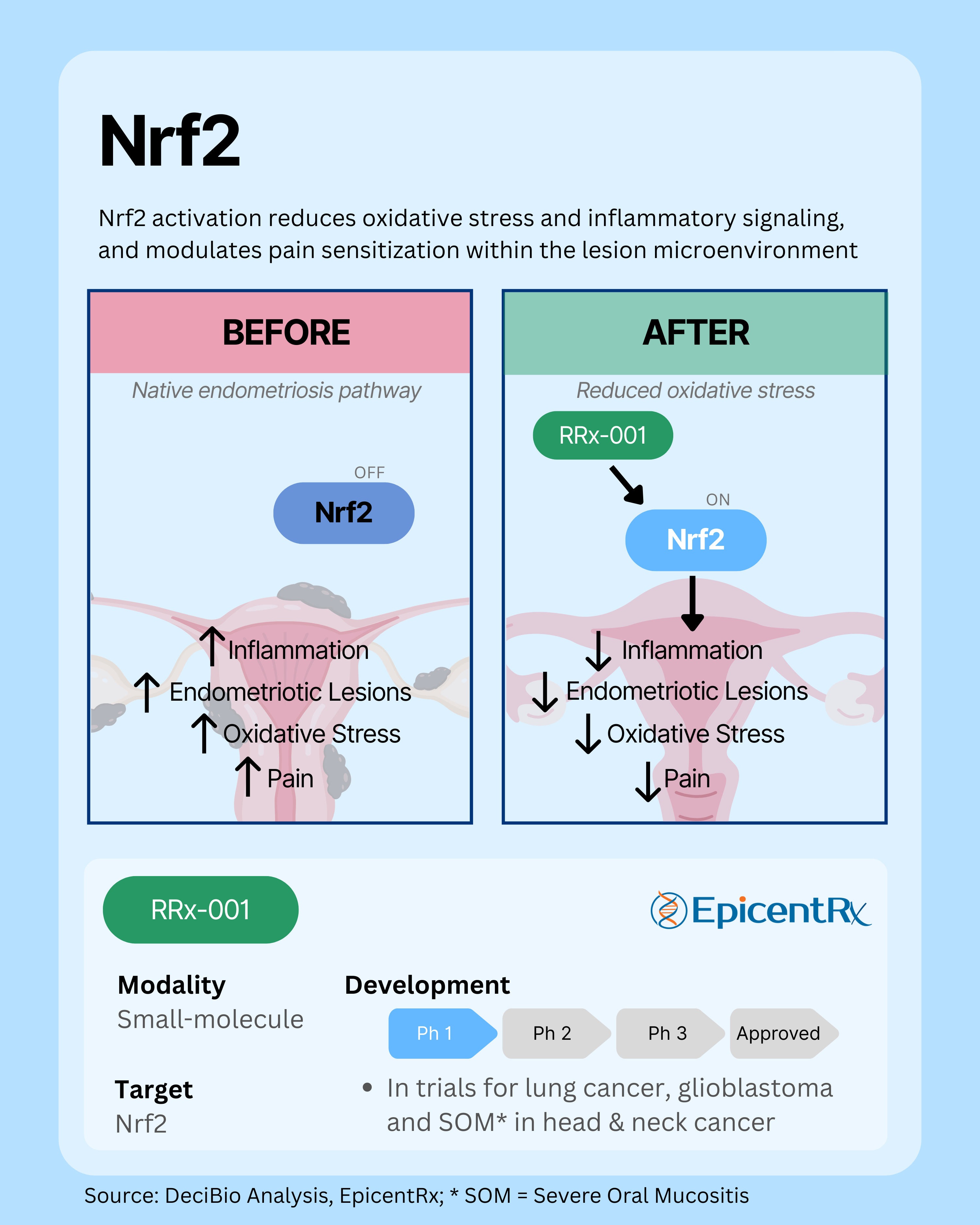
2. Nrf2: Restoring Antioxidant and Anti-Inflammatory Balance
The Nrf2 pathway is a master regulator of cellular antioxidant and anti-inflammatory responses, but its activity is often impaired in endometriosis, leading to oxidative stress, chronic inflammation, and pain sensitization within the lesion microenvironment. Reduced Nrf2 signaling contributes to lesion growth and disease progression. By reactivating this pathway, emerging therapies aim to restore redox balance, dampen inflammation and pain, and limit abnormal vascular remodeling, potentially modifying the course of the disease rather than just alleviating symptoms, all without suppressing systemic hormone levels.
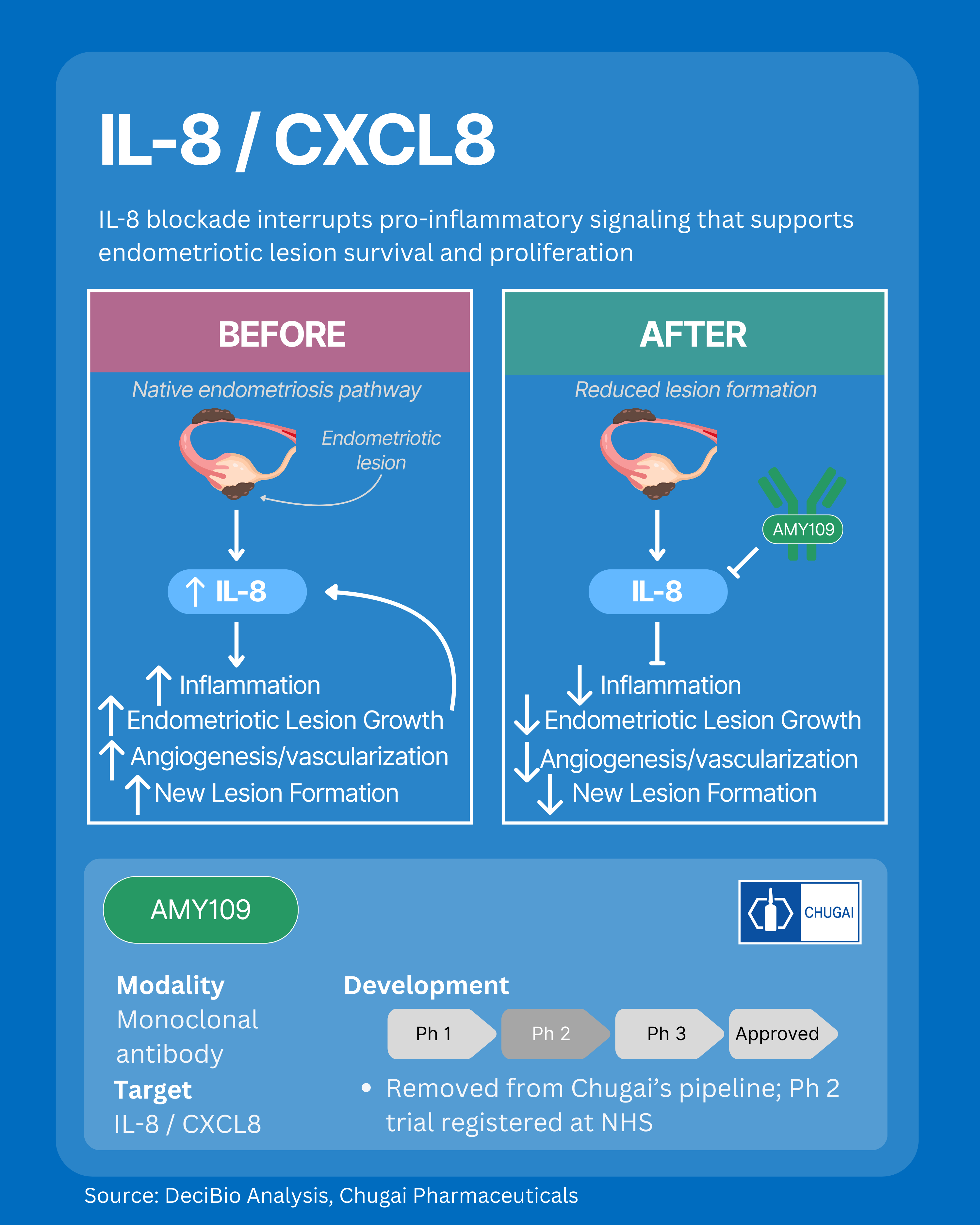
3. IL-8: Disrupting Inflammation-Driven Lesion Survival
IL-8 is a potent pro-inflammatory chemokine that drives endometriosis lesion growth, angiogenesis, and vascularization within the disease microenvironment. Elevated IL-8 levels in lesions and peritoneal fluid recruit immune cells, activate endothelial cells, and stimulate new blood vessel formation, directly supporting lesion survival and expansion. IL-8 also inhibits apoptosis and amplifies chronic inflammation, enabling persistent lesion formation and disease progression. Targeting IL-8 signaling might interrupt these disease-driving pathways and modify underlying endometriosis disease biology, without systemic hormone suppression. However, with the leading asset no longer in an active pharma pipeline, translating this mechanism into clinical success may be delayed.
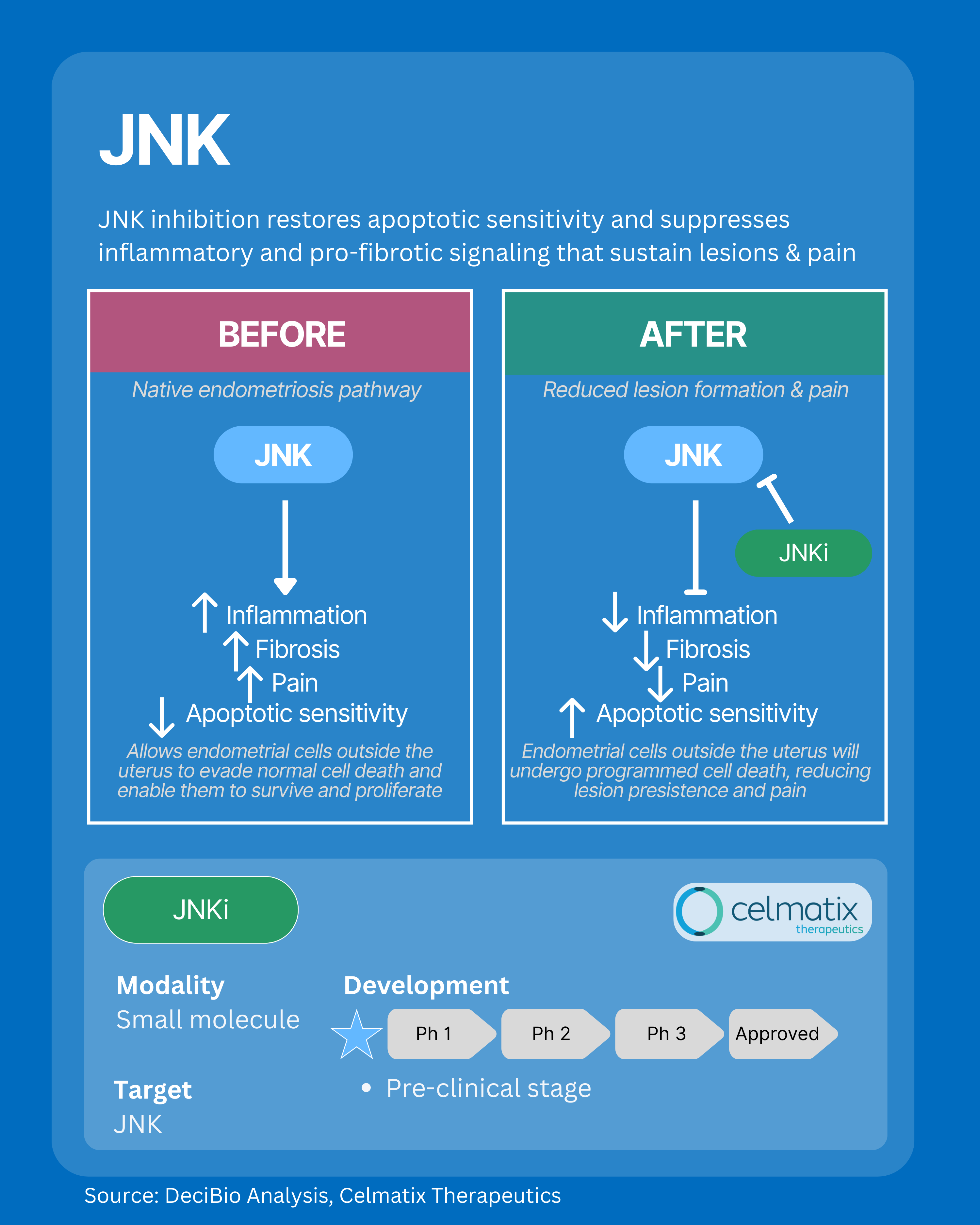
4. JNK: Interrupting Fibrosis, Inflammation, and Pain Signaling
The JNK pathway is a central regulator of inflammation, immune activation, and pain signaling within endometriosis lesions. Overactivation of JNK in the lesion microenvironment disrupts apoptosis, or cell death, allowing abnormal cells to survive, and drives the development of dense, fibrotic tissue through pro-fibrotic signaling. By targeting JNK, emerging treatments aim to restore apoptotic sensitivity and interrupt fibrotic remodeling, dampening chronic inflammation, fibrosis, and pain at the source, and offering disease-modifying potential independent of systemic hormone suppression.
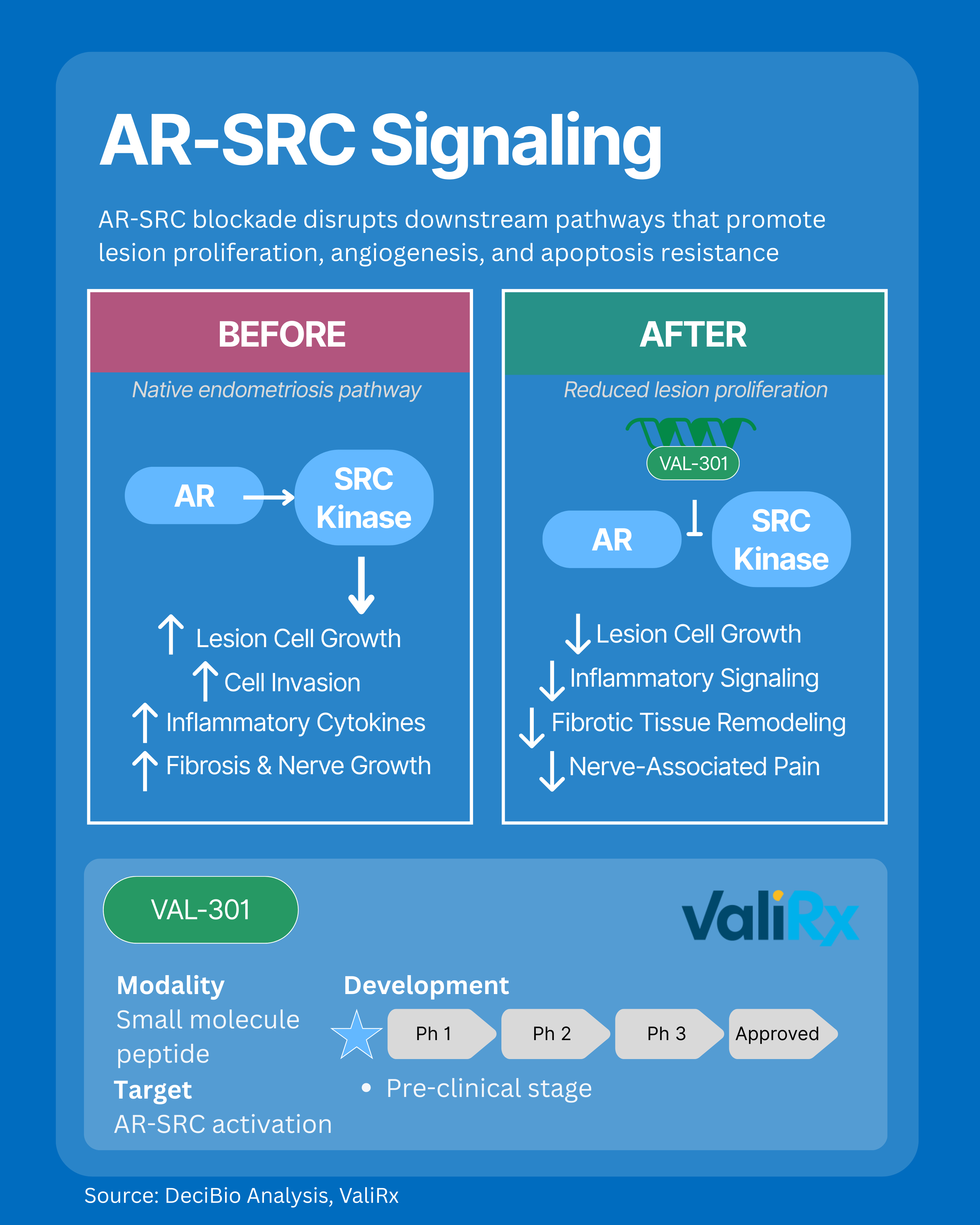
5. AR-SRC Signaling: Addressing Proliferation, Fibrosis, and Nerve-Driven Pain
The AR-SRC signaling axis is a key driver of lesion proliferation, angiogenesis, apoptosis resistance, and fibrosis in endometriosis. Overactivation of this pathway fuels abnormal cell growth, blood vessel formation, fibrotic remodeling, invasion of surrounding tissues, and nerve growth, leading to nerve associated pain and persistent inflammation via heightened cytokine release. By targeting AR-SRC signaling, new therapies aim to halt lesion expansion, restore programmed cell death, reduce fibrosis, temper nerve-driven pain, and block aggressive cell invasion, addressing biological root causes of endometriosis without suppressing hormones systemically.
The Missing Piece: Precision Diagnostics
At DeciBio, we believe progress in therapeutics must be matched by innovation in diagnostics. Precision treatments can only succeed if patients are diagnosed earlier, more accurately, and in ways that reflect underlying biology.
We can’t treat what we can’t detect—and without better diagnostic tools, even the most promising therapies will struggle to reach the patients who could benefit most.
Looking Ahead
The shift toward mechanism-based, disease-modifying treatment in endometriosis is already underway. While challenges remain. across funding, clinical evidence generation, and access, the growing focus on molecular pathways represents meaningful momentum for a disease that has long been overlooked.
The more we understand the biology of endometriosis, the closer we get to durable, personalized treatment options that can truly change lives.
To dive deeper into these topics, you can read our full Q&A with DeciBio experts and industry leaders on building the precision medicine bridge in endometriosis here: Endometriosis & Building the Precision Medicine Bridge.
Comments and opinions expressed by interviewees are their own and do not represent or reflect the opinions, policies, or positions of DeciBio Consulting or have its endorsement. Note: DeciBio Consulting, its employees or owners, or our guests may hold assets discussed in this article/episode. This article/blog/episode does not provide investment advice, and is intended for informational and entertainment purposes only. You should do your own research and make your own independent decisions when considering any financial transactions.




.png)

.png)

